PUTNEY — CPR Therapeutics, Inc., a medical device startup company based here, has received a $1.6 million award of federal funding for a project to help save the lives of people who need cardio-pulmonary resuscitation.
The funding is from the National Institutes of Health (NIH) National Heart Lung and Blood Institute (NHLBI) Direct-To-Phase II Small Business Innovation Research Award. It will go toward community efforts to build “A Multimodal Integrated System for Improved Cardiopulmonary Resuscitation.”
“We are over the moon!” says Dr. Norman Paradis, CPR Therapeutics founder/CEO and one of the leading experts in the world regarding ventricular fibrillation.
“We are tremendously excited to have received this level of support from one of the world's most prestigious medical research institutions,” he said. “Direct-to-Phase II means that the NIH-NHLBI reviewers agree that we have made significant progress already.”
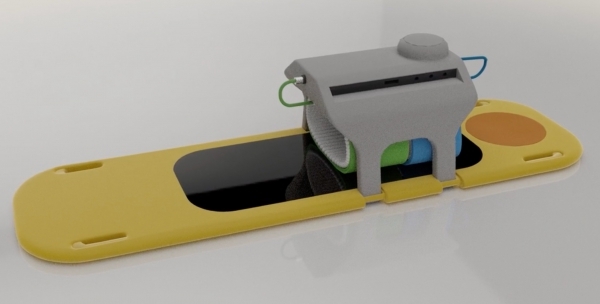
CPR Therapeutics has been developing the first advanced-technology automated CPR system that integrates and synchronizes multiple technological innovations into a single system that can be easily applied under emergency conditions.
Company officials believe the technology will, for the first time, demonstrate clinically significant improvements in intact survival after sudden cardiac arrest, the leading cause of death in Western countries.
Currently, no device is more effective than manual CPR. Intact survival after sudden cardiac arrest - that is, rates of patients making it through this health crisis with no change in the neurological function - is less than 10 percent in most communities.
The project and studies will be conducted in collaboration with The Johns Hopkins Hospital in Baltimore, Maryland.
Henry Halperin M.D., the company's chief scientific officer and professor of cardiology and biomedical engineering at Johns Hopkins, says, “This level of support should be sufficient for us to complete research and development of our first-generation multimodal CPR device. The NHLBI review process is rigorous, so I can't think of a better validation of our underlying science, our efforts so far, and the current development and business plans.”
The NIH support will accelerate the company's preclinical research and development and device prototyping, resulting in a first clinical system ready to submit to the Food and Drug Administration for clearance.
“Our whole team could not be more excited,” Paradis says.
The NIH windfall of support comes just a month after CPR Therapeutics received a $256,000 National Science Foundation (NSF) Small Business Technology Transfer (STTR) Phase I award for the same project.
“We are excited to have received this validation of our efforts from one of the most prestigious scientific organizations in the world,” Paradis says of the NSF award. “The NSF has an extraordinary record of identifying and supporting innovations that eventually reach the bedside and improve patient outcomes.”
Paradis, an emergency physician with significant experience in private-sector biomedical device development, leads a large collaboration developing a shock device for the Department of Defense. A professor of medicine at the Geisel School of Medicine at Dartmouth College, he holds patents on multiple innovations.
“This support will significantly advance our ongoing preclinical development of multimodal CPR,” adds Halperin. “In particular, it will allow us to focus on optimizing the synergies between the multiple pump mechanisms and refin[ing] the synchronization with electrical counter-shock.”
Halperin is a cardiologist and co-inventor of circumferential chest compressions, with more than 180 peer-reviewed publications. He is a Fellow of National Academy of Inventors, with more than 75 patents. He is past chair of the National American Heart Association ACLS Subcommittee.
Studies supported by the awards are to begin immediately and will accelerate the company's plan to commercialize an in-hospital version of its Vest-CPR System.
Improving survival
“I'm the doctor on this project, but the engineers on this project are pretty impressive,” says Paradis.
It was in the late '80s or early '90s when a group - “we were all pretty junior,” says Paradis - at Johns Hopkins discovered a better way of doing CPR than squeezing in the middle of the chest.
“That fractures ribs 70 percent of the time, and it doesn't move much blood,” Paradis says. “I was collaborating with them, so I would visit there. I called around and said, 'If we didn't do this, we'd all be in the nursing home someday not having done it.'”
Paradis and his group found that “if you squeeze the whole chest, you don't break many ribs and you can move maybe twice as much blood.”
“It was a pretty exciting project but when they went to move it to an actual medical device, the batteries weren't adequate for the force that was needed, so they abandoned it,” he says.
“We kept working on it and, obviously, batteries have gotten better,” Paradise says.
“About six years ago we approached all the medical companies, but it looked like too challenging a problem for them,” he recounted.
One company ultimately offered a small investment and a small group of angel investors came forth and “that got us started,” says Paradis.
The company hopes a version of the device will be developed for hospitals in about two years.
Miniaturizing the device - which is pneumatic, working off compressed air - will take at least another year or two after that.
“There has never been a successful new device for CPR,” Paradis says. “Medicine is probably a numbers game. Every disease claims it is the biggest killer, but it's generally accepted that outside of Covid, sudden cardiac death is the largest killer in western countries.”
“Medical professionals say three jumbo jets a day daily - 1,000 people in the United States - die daily,” he cautions. “If you're having a heart attack and die suddenly that day, it's really cardiac arrest. Worldwide, some estimate the number at 5,000.”
“The survival rate on TV shows and movies is 90 percent, but in regular life, it's less than 10 percent,” he continued. “And in rural communities, it's way less than 5 percent because the transport times are so long.”
Paradis says the new piece of equipment is “not a complicated device.”
“It looks like a much bigger version of a blood pressure cuff,” he says, adding it can be attached in less than 20 seconds.
The hope is for it to be available one day in airports as well, because automated defibrillators can be built into the device to do both jobs.
Paradis is grateful for the national support.
“I have put this idea in front of hundreds of investors, and they all understand it's the largest killer in western countries, that our solution works, and that in the end most of the investment is going to come from the government because it looked too risky to private investment,” he says.
“I hear all the time that the government doesn't do anything, but I say really, they took a flyer on us,” Paradis observes.